Health
Kymera Drug KT-621 Achieves Success in Eczema Trial, Challenging Dupixent
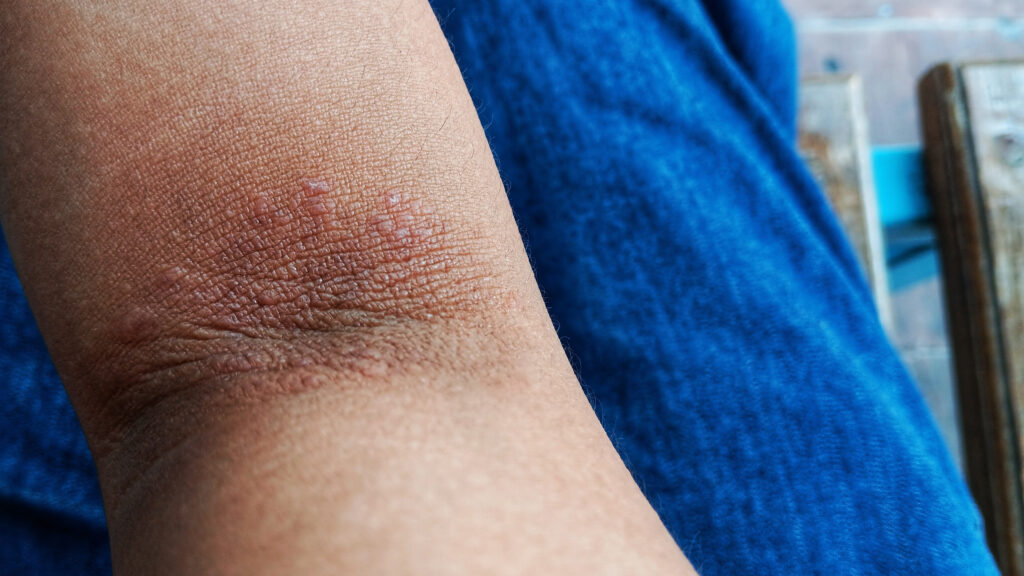
Kymera Therapeutics announced significant progress in its quest to compete with the well-established eczema treatment, Dupixent, developed by Sanofi and Regeneron. In a recent Phase 1 clinical trial known as the BroADen study, the company’s experimental oral drug, KT-621, demonstrated promising results in patients suffering from moderate-to-severe eczema.
The trial enrolled 22 participants who received KT-621 for four weeks, followed by a two-week observation period. According to results, the patients experienced an average reduction of 63% in disease severity, as measured by the Eczema Area and Severity Index (EASI) score. This outcome exceeded expectations within the financial community, as noted by analysts at Leerink Partners.
Market Context and Competitive Landscape
Based in Watertown, Massachusetts, Kymera Therapeutics currently holds a market capitalization of approximately $4.8 billion. The company is positioning KT-621 as a formidable competitor to Dupixent, which generated more than $14 billion in revenue last year. This antibody-based therapy has received approval for various conditions, including eczema, asthma, and chronic obstructive pulmonary disease, owing to its ability to inhibit the inflammatory cytokines IL-4 and IL-13.
Dupixent has established itself as a leading treatment in the dermatological space, prompting Kymera to accelerate the development of its own therapeutic options. The success of KT-621 may hold the potential to reshape treatment paradigms for patients with atopic dermatitis, a condition impacting millions globally.
The results from the BroADen study are particularly significant as they come at a time when the demand for effective eczema treatments is on the rise. As the prevalence of atopic dermatitis continues to grow, innovations in the treatment landscape could offer new hope to those affected.
Looking Ahead
Kymera intends to build on the momentum of these trial results as it advances KT-621 through further stages of clinical development. The company’s strategy will likely focus on expanding its clinical trials to a larger patient population while continuing to gather data on the long-term efficacy and safety of the drug.
In a competitive field dominated by established players, Kymera’s early success could pave the way for significant advancements in eczema therapy. Investors and healthcare professionals alike will be closely monitoring the upcoming stages of the BroADen study and the company’s future announcements regarding KT-621.
As the pharmaceutical industry evolves, Kymera Therapeutics stands poised to challenge the status quo in eczema treatment, potentially offering patients a new and effective option.
-

 Top Stories1 month ago
Top Stories1 month agoUrgent Update: Tom Aspinall’s Vision Deteriorates After UFC 321
-

 Science1 month ago
Science1 month agoUniversity of Hawaiʻi Joins $25.6M AI Project to Enhance Disaster Monitoring
-

 Health2 months ago
Health2 months agoMIT Scientists Uncover Surprising Genomic Loops During Cell Division
-

 Top Stories2 months ago
Top Stories2 months agoAI Disruption: AWS Faces Threat as Startups Shift Cloud Focus
-

 Science2 months ago
Science2 months agoTime Crystals Revolutionize Quantum Computing Potential
-

 Entertainment2 months ago
Entertainment2 months agoDiscover the Full Map of Pokémon Legends: Z-A’s Lumiose City
-

 Entertainment2 months ago
Entertainment2 months agoParenthood Set to Depart Hulu: What Fans Need to Know
-

 World2 months ago
World2 months agoHoneywell Forecasts Record Business Jet Deliveries Over Next Decade
-

 Top Stories2 months ago
Top Stories2 months agoGOP Faces Backlash as Protests Surge Against Trump Policies
-

 Politics2 months ago
Politics2 months agoJudge Signals Dismissal of Chelsea Housing Case Citing AI Flaws
-

 Health2 months ago
Health2 months agoMaine Insurers Cut Medicare Advantage Plans Amid Cost Pressures
-

 Sports2 months ago
Sports2 months agoYoshinobu Yamamoto Shines in Game 2, Leading Dodgers to Victory