Science
Spero and GSK Reveal Promising Phase 3 Results for Oral Antibiotic

Spero Therapeutics, Inc. and GSK plc announced promising results from the pivotal Phase 3 PIVOT-PO trial of tebipenem HBr, aimed at treating complicated urinary tract infections (cUTIs), including pyelonephritis. These findings were revealed on October 20, 2025, during a late-breaking oral abstract session at IDWeek 2025, held in Atlanta, Georgia.
The PIVOT-PO trial assessed the efficacy and safety of tebipenem HBr, which has the potential to become the first oral carbapenem antibiotic. This class of antibiotics is traditionally administered intravenously, making the oral formulation a significant advancement in treatment options for patients.
In the trial, tebipenem HBr demonstrated a notable efficacy rate, which could change the management landscape for cUTIs. The study involved a diverse group of participants, ensuring that the results reflect a broad patient demographic. The positive outcome suggests that tebipenem HBr could provide a critical alternative for patients who require effective treatment for urinary tract infections.
The results presented at IDWeek 2025 highlight the potential for a new oral treatment option that may simplify patient care. Spero Therapeutics and GSK are now preparing for the next steps in the regulatory process, which includes discussions with health authorities to potentially expedite the approval of tebipenem HBr.
Spero Therapeutics’ Chief Executive Officer, Dr. Ankit Mahadevia, expressed optimism about the results, stating, “The findings from the PIVOT-PO trial underline our commitment to addressing unmet needs in infectious disease treatment.” This sentiment is echoed by GSK’s representatives, who recognize the importance of advancing effective therapies in the face of increasing antibiotic resistance.
The trial’s success not only underscores the viability of tebipenem HBr but also highlights the importance of continued innovation in antibiotic development, particularly as healthcare systems worldwide grapple with rising rates of antibiotic-resistant infections.
Both companies will continue to monitor the drug’s performance through post-marketing studies, should it receive regulatory approval. The PIVOT-PO trial results mark a potential turning point in the treatment of cUTIs, providing hope to patients and healthcare providers alike.
As the pharmaceutical industry pushes for advancements in antibiotic therapies, the implications of the PIVOT-PO trial extend beyond just one medication. It raises awareness regarding the critical need for new treatments in an era where antibiotic resistance poses significant challenges to public health.
The journey towards regulatory approval is now in motion, and the medical community eagerly anticipates further developments regarding tebipenem HBr and its role in combating complex urinary tract infections.
-

 Top Stories1 month ago
Top Stories1 month agoUrgent Update: Tom Aspinall’s Vision Deteriorates After UFC 321
-
 Health1 month ago
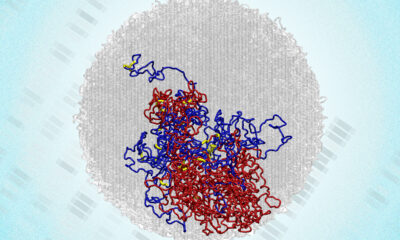
Health1 month agoMIT Scientists Uncover Surprising Genomic Loops During Cell Division
-

 Science4 weeks ago
Science4 weeks agoUniversity of Hawaiʻi Joins $25.6M AI Project to Enhance Disaster Monitoring
-

 Top Stories1 month ago
Top Stories1 month agoAI Disruption: AWS Faces Threat as Startups Shift Cloud Focus
-

 Science2 months ago
Science2 months agoTime Crystals Revolutionize Quantum Computing Potential
-

 World2 months ago
World2 months agoHoneywell Forecasts Record Business Jet Deliveries Over Next Decade
-

 Entertainment1 month ago
Entertainment1 month agoDiscover the Full Map of Pokémon Legends: Z-A’s Lumiose City
-

 Top Stories2 months ago
Top Stories2 months agoGOP Faces Backlash as Protests Surge Against Trump Policies
-

 Entertainment2 months ago
Entertainment2 months agoParenthood Set to Depart Hulu: What Fans Need to Know
-

 Politics2 months ago
Politics2 months agoJudge Signals Dismissal of Chelsea Housing Case Citing AI Flaws
-

 Sports2 months ago
Sports2 months agoYoshinobu Yamamoto Shines in Game 2, Leading Dodgers to Victory
-

 Health2 months ago
Health2 months agoMaine Insurers Cut Medicare Advantage Plans Amid Cost Pressures







