Science
New Nasal Vaccine Promises to Change Respiratory Disease Prevention
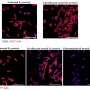
A research team from Trinity College Dublin has developed a groundbreaking nasal vaccine that could significantly improve prevention strategies for respiratory infections. The study, published in Nature Microbiology, reveals that their innovative vaccine, which utilizes antibiotic-inactivated Bordetella pertussis (AIBP), not only prevents severe disease but also reduces bacterial transmission. This advancement addresses a crucial need in the quest for effective respiratory vaccines.
Led by Professor Kingston Mills and Dr. Davoud Jazayeri from the university’s School of Biochemistry and Immunology, the AIBP vaccine introduces a needle-free mucosal platform designed to stimulate lasting immunity directly at the site of infection. This approach could revolutionize the prevention of whooping cough and enhance the overall landscape of respiratory bacterial vaccines.
“We’ve applied our understanding of protective immune pathways to engineer a fundamentally different kind of vaccine,” said Professor Mills. “By stimulating immunity where infections begin, at the respiratory mucosa, we can offer stronger protection and potentially interrupt community transmission.”
Current whooping cough vaccines have been effective in protecting infants from severe illness; however, they do not prevent bacterial colonization in the nose and throat. This limitation has contributed to the recent resurgence of pertussis, even in populations with high vaccination rates. The findings from Trinity College highlight the urgent commercial and clinical demand for next-generation vaccines.
The AIBP vaccine is delivered intranasally, a method that activates a unique T-cell-driven mucosal immune response. This response is critical for providing protection to both the lungs and the upper respiratory tract while minimizing the risk of unwanted systemic inflammation. In preclinical studies, AIBP demonstrated complete protection against infections in both the lungs and nasal cavity, outperforming existing acellular pertussis vaccines.
These promising results suggest that AIBP could serve as a standalone next-generation pertussis vaccine. Additionally, its platform may be adaptable to combat other pathogens, including Staphylococcus aureus, Streptococcus pneumoniae, Mycoplasma pneumoniae, and Mycobacterium tuberculosis.
The implications of this research are significant, as improving vaccination strategies against respiratory diseases remains a critical global health priority. Future studies will further explore the potential of this innovative vaccine technology in addressing a range of infectious diseases.
For more detailed information, refer to the study: Seyed Davoud Jazayeri et al, “Respiratory immunization using antibiotic-inactivated Bordetella pertussis confers T cell-mediated protection against nasal infection in mice,” published in Nature Microbiology (2025). DOI: 10.1038/s41564-025-02166-6.
-

 Top Stories1 month ago
Top Stories1 month agoUrgent Update: Tom Aspinall’s Vision Deteriorates After UFC 321
-

 Health1 month ago
Health1 month agoMIT Scientists Uncover Surprising Genomic Loops During Cell Division
-

 Science4 weeks ago
Science4 weeks agoUniversity of Hawaiʻi Joins $25.6M AI Project to Enhance Disaster Monitoring
-

 Top Stories1 month ago
Top Stories1 month agoAI Disruption: AWS Faces Threat as Startups Shift Cloud Focus
-

 Science2 months ago
Science2 months agoTime Crystals Revolutionize Quantum Computing Potential
-

 World2 months ago
World2 months agoHoneywell Forecasts Record Business Jet Deliveries Over Next Decade
-

 Entertainment1 month ago
Entertainment1 month agoDiscover the Full Map of Pokémon Legends: Z-A’s Lumiose City
-

 Top Stories2 months ago
Top Stories2 months agoGOP Faces Backlash as Protests Surge Against Trump Policies
-

 Entertainment2 months ago
Entertainment2 months agoParenthood Set to Depart Hulu: What Fans Need to Know
-

 Politics2 months ago
Politics2 months agoJudge Signals Dismissal of Chelsea Housing Case Citing AI Flaws
-

 Sports2 months ago
Sports2 months agoYoshinobu Yamamoto Shines in Game 2, Leading Dodgers to Victory
-

 Health2 months ago
Health2 months agoMaine Insurers Cut Medicare Advantage Plans Amid Cost Pressures







