Science
New Algorithms Illuminate Propane-to-Propylene Conversion Process
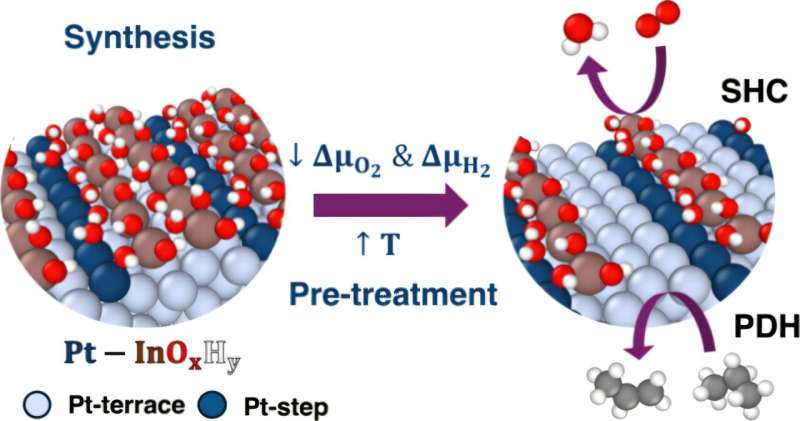
A team of researchers from the University of Rochester has made significant strides in understanding the conversion of propane to propylene, a crucial process for producing many everyday products. Their innovative algorithms reveal the atomic-level interactions that occur during this transformation, enhancing efficiency and potentially reducing costs for manufacturers.
In a study published on November 13, 2025, in the Journal of the American Chemical Society, researchers describe how nanoscale catalysts can streamline this chemical process. Previously, a 2021 study highlighted the potential of using tandem nanoscale catalysts to consolidate multiple steps into a single reaction, but the underlying mechanisms remained elusive.
Deciphering Catalytic Processes at the Atomic Level
The research team, led by assistant professor Siddharth Deshpande, focused on utilizing algorithms to analyze the complex chemistry involved in the catalytic reactions. “There are so many different possibilities of what’s happening at the catalytic active sites, so we need an algorithmic approach to very easily yet logically screen through the large amount of possibilities that exist and focus on the most important ones,” Deshpande explained.
By refining their algorithms, Deshpande and his chemical engineering Ph.D. student, Snehitha Srirangam, conducted a comprehensive analysis of the metallic and oxide phases involved in the reaction. Their findings revealed unexpected behavior: the oxide selectively grows around defective metal sites, which is critical for the catalyst’s stability.
Implications for Future Chemical Production
This new understanding could revolutionize how companies approach the production of propylene and other industrial materials. According to Deshpande, leveraging this knowledge can lead to more strategic and efficient production methods, moving away from traditional trial-and-error techniques that have dominated the industry for decades.
“Our approach is very general and can open the doors to understanding many of these processes that have remained an enigma for decades,” Deshpande stated. He emphasized that while these chemical processes are widely used, the detailed mechanisms have been poorly understood until now.
The researchers believe their work not only sheds light on the propane-to-propylene conversion but also holds potential for other chemical reactions, such as methanol synthesis, which is essential for products ranging from paints to fuel cells.
With this breakthrough, the team at the University of Rochester is paving the way for advancements that could enhance the efficiency of numerous industrial processes, ultimately benefiting manufacturers and consumers alike.
-

 Top Stories1 month ago
Top Stories1 month agoUrgent Update: Tom Aspinall’s Vision Deteriorates After UFC 321
-

 Health1 month ago
Health1 month agoMIT Scientists Uncover Surprising Genomic Loops During Cell Division
-

 Science4 weeks ago
Science4 weeks agoUniversity of Hawaiʻi Joins $25.6M AI Project to Enhance Disaster Monitoring
-

 Top Stories1 month ago
Top Stories1 month agoAI Disruption: AWS Faces Threat as Startups Shift Cloud Focus
-

 Science2 months ago
Science2 months agoTime Crystals Revolutionize Quantum Computing Potential
-

 World2 months ago
World2 months agoHoneywell Forecasts Record Business Jet Deliveries Over Next Decade
-

 Entertainment1 month ago
Entertainment1 month agoDiscover the Full Map of Pokémon Legends: Z-A’s Lumiose City
-

 Top Stories2 months ago
Top Stories2 months agoGOP Faces Backlash as Protests Surge Against Trump Policies
-

 Entertainment2 months ago
Entertainment2 months agoParenthood Set to Depart Hulu: What Fans Need to Know
-

 Politics2 months ago
Politics2 months agoJudge Signals Dismissal of Chelsea Housing Case Citing AI Flaws
-

 Sports2 months ago
Sports2 months agoYoshinobu Yamamoto Shines in Game 2, Leading Dodgers to Victory
-

 Health2 months ago
Health2 months agoMaine Insurers Cut Medicare Advantage Plans Amid Cost Pressures








