Health
Family Raises $6M for FDA-Approved Treatment of Rare Disorder

A family from Natchitoches, Louisiana, is mobilizing to raise $6 million for FDA-approved treatment for their daughter, Lydia, who has been diagnosed with a rare condition known as Sanfilippo syndrome B, often referred to as “childhood dementia.” The urgency of their fundraising effort stems from a serendipitous discovery made by Lydia’s grandmother, Cindy Weaver, who spotted a TikTok video featuring a child with similar physical traits and symptoms.
Initially, Lydia’s mother, Morgan Rachal, believed her daughter was healthy, aside from common issues like ear infections and sleep disturbances. However, after seeing the TikTok, which showcased another child with the same thick eyebrows—indicative of Sanfilippo syndrome—Morgan consulted with Lydia’s pediatrician. Tests confirmed Lydia’s diagnosis just weeks later, shaking the family to its core.
Understanding Sanfilippo Syndrome
Sanfilippo syndrome is a neurodegenerative disorder that leads to a gradual loss of skills, including the ability to walk and talk. Children with this condition often display distinct physical features, such as thick eyebrows and full lips. Lydia’s type, B, is caused by a defect in the NAGLU gene, which impairs the body’s ability to break down certain sugars, leading to toxic accumulation in the brain.
The diagnosis has profound implications for Lydia’s future. Doctors informed Morgan and her husband, Kirk Rachal, that there is currently no cure for Sanfilippo syndrome and advised them to focus on making memories with their daughter. “They call it childhood dementia,” Morgan explained. “Her joy right now will be taken away if she doesn’t get into treatment.”
Raising Funds for Hope
Determined not to surrender to despair, Morgan joined forces with 14 other families, all of whom are facing similar challenges with children diagnosed with Sanfilippo syndrome. Together, they are aiming to raise the necessary funds for enzyme replacement therapy, a treatment currently under development and awaiting FDA approval. This therapy aims to replace the missing enzymes in children with Sanfilippo, potentially offering a lifeline.
The families have already raised $1.6 million and are hopeful that the drug will receive approval by 2027. Morgan has set a target to collect $3.8 million by December 1, 2025, with the remaining funds needed by the following year. “This is our last chance to save our daughter,” she stated. “I am so grateful for everyone who is helping.”
The Cure Sanfilippo Foundation has been instrumental in spreading awareness and providing resources for families affected by this rare disorder. According to the foundation, enzyme replacement therapy is a promising avenue of treatment but remains in clinical trials. As Morgan and her community rally support, they remain optimistic that their efforts will lead to tangible results.
For those interested in contributing to Lydia’s treatment fund, donations can be made through the GoFundMe campaign titled “Donate to Save Lydia,” organized by Morgan Rachal.
-

 Top Stories1 month ago
Top Stories1 month agoUrgent Update: Tom Aspinall’s Vision Deteriorates After UFC 321
-
 Health1 month ago
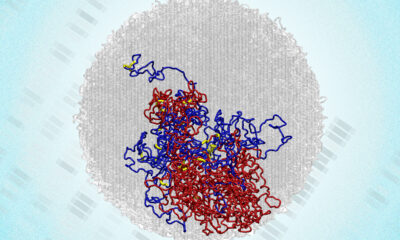
Health1 month agoMIT Scientists Uncover Surprising Genomic Loops During Cell Division
-

 Science4 weeks ago
Science4 weeks agoUniversity of Hawaiʻi Joins $25.6M AI Project to Enhance Disaster Monitoring
-

 Top Stories1 month ago
Top Stories1 month agoAI Disruption: AWS Faces Threat as Startups Shift Cloud Focus
-

 Science2 months ago
Science2 months agoTime Crystals Revolutionize Quantum Computing Potential
-

 World2 months ago
World2 months agoHoneywell Forecasts Record Business Jet Deliveries Over Next Decade
-

 Entertainment1 month ago
Entertainment1 month agoDiscover the Full Map of Pokémon Legends: Z-A’s Lumiose City
-

 Top Stories2 months ago
Top Stories2 months agoGOP Faces Backlash as Protests Surge Against Trump Policies
-

 Entertainment2 months ago
Entertainment2 months agoParenthood Set to Depart Hulu: What Fans Need to Know
-

 Politics2 months ago
Politics2 months agoJudge Signals Dismissal of Chelsea Housing Case Citing AI Flaws
-

 Sports2 months ago
Sports2 months agoYoshinobu Yamamoto Shines in Game 2, Leading Dodgers to Victory
-

 Health2 months ago
Health2 months agoMaine Insurers Cut Medicare Advantage Plans Amid Cost Pressures








