Science
New Method Cuts Costs to Produce Copper-64 for Medical Imaging
The production of the copper-64 isotope, crucial for medical imaging and cancer therapy, has become more accessible and cost-effective, thanks to research from the Vienna University of Technology (TU Wien). A team led by researchers Veronika Rosecker and Martin Pressler has demonstrated a novel approach using neutron irradiation, reducing reliance on traditional methods that require expensive cyclotron technology.
Traditionally, Cu-64 is produced by bombarding nickel atoms with protons. This method is complex and costly, as it necessitates enriched nickel-64 (Ni-64), which is itself a rare isotope. The conventional process transforms nickel into copper by ejecting a neutron when the nickel nucleus absorbs a proton. While effective, the expenses associated with cyclotrons and the need for enriched isotopes have limited widespread application.
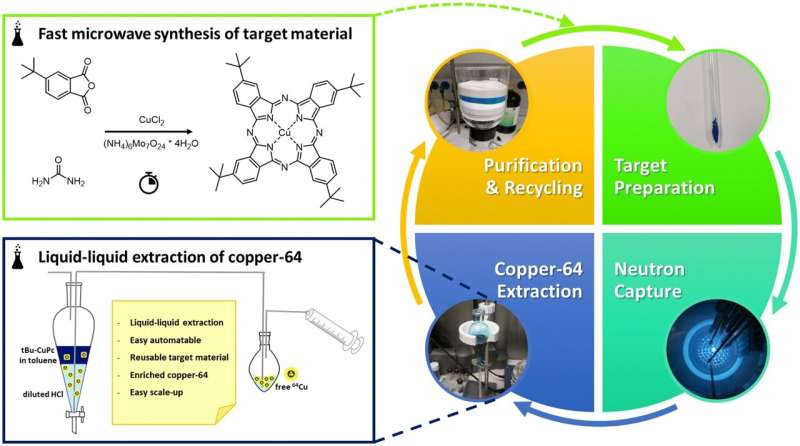
The breakthrough at TU Wien involves converting copper-63 (Cu-63) into Cu-64 by leveraging a technique known as recoil chemistry. This innovative approach allows researchers to utilize a research reactor to produce Cu-64 with significantly lower costs and simpler logistics.
Understanding Recoil Chemistry
Copper isotopes differ in neutron count; Cu-63, the most stable variant, contains 34 neutrons, while Cu-64 has one additional neutron and is radioactive with a half-life of about 13 hours. This decay rate makes Cu-64 particularly valuable for medical applications, as it maintains stability during transportation to treatment sites while minimizing radiation exposure for patients.
In their research, the team discovered that when Cu-63 is irradiated with neutrons, Cu-64 nuclei are generated. However, the challenge lies in separating the newly formed Cu-64 from the existing Cu-63. Pressler explained that without a suitable method, the result is a mixture dominated by ordinary copper.
The solution lies in the recoil effect produced during neutron absorption. When a Cu-63 atom captures a neutron and becomes Cu-64, it releases energy in the form of gamma radiation. This emission causes the atom to recoil, allowing it to escape from its molecular structure, while the remaining Cu-63 stays within the molecule. This separation method enhances the efficiency of extracting Cu-64.
Developing a Suitable Molecule
Identifying an appropriate molecule for this process posed significant challenges. The selected molecule had to withstand the harsh conditions within a nuclear reactor while remaining soluble for efficient recovery of the isotope. The researchers achieved this by utilizing a metal-organic complex resembling heme, the molecule found in human blood.
Previous studies had examined similar complexes, but they struggled with solubility. The TU Wien team chemically modified the complex to ensure it could dissolve, allowing for effective recovery of Cu-64 after the neutron irradiation process.
This method offers significant advantages over traditional approaches. It can be automated, allows for the reuse of the molecules, and operates using a standard research reactor rather than requiring specialized cyclotron facilities.
The findings from this study were published in the journal Dalton Transactions on March 15, 2025. This advancement not only promises to lower production costs for Cu-64 but also enhances its accessibility for medical applications, potentially improving imaging and treatment options for patients worldwide.
For more detailed insights into this innovative method, refer to the publication by Martin Pressler et al., titled “Fast and easy reactor-based production of copper-64 with high molar activities using recoil chemistry.”
-

 Top Stories1 month ago
Top Stories1 month agoUrgent Update: Tom Aspinall’s Vision Deteriorates After UFC 321
-

 Science1 month ago
Science1 month agoUniversity of Hawaiʻi Joins $25.6M AI Project to Enhance Disaster Monitoring
-

 Health2 months ago
Health2 months agoMIT Scientists Uncover Surprising Genomic Loops During Cell Division
-

 Top Stories2 months ago
Top Stories2 months agoAI Disruption: AWS Faces Threat as Startups Shift Cloud Focus
-

 Science2 months ago
Science2 months agoTime Crystals Revolutionize Quantum Computing Potential
-

 Entertainment2 months ago
Entertainment2 months agoParenthood Set to Depart Hulu: What Fans Need to Know
-

 Top Stories2 months ago
Top Stories2 months agoGOP Faces Backlash as Protests Surge Against Trump Policies
-

 Entertainment2 months ago
Entertainment2 months agoDiscover the Full Map of Pokémon Legends: Z-A’s Lumiose City
-

 World2 months ago
World2 months agoHoneywell Forecasts Record Business Jet Deliveries Over Next Decade
-

 Politics2 months ago
Politics2 months agoJudge Signals Dismissal of Chelsea Housing Case Citing AI Flaws
-

 Health2 months ago
Health2 months agoMaine Insurers Cut Medicare Advantage Plans Amid Cost Pressures
-

 Sports2 months ago
Sports2 months agoYoshinobu Yamamoto Shines in Game 2, Leading Dodgers to Victory