Science
Researchers Unveil Innovative One-Pot Method for Aryne Precursors
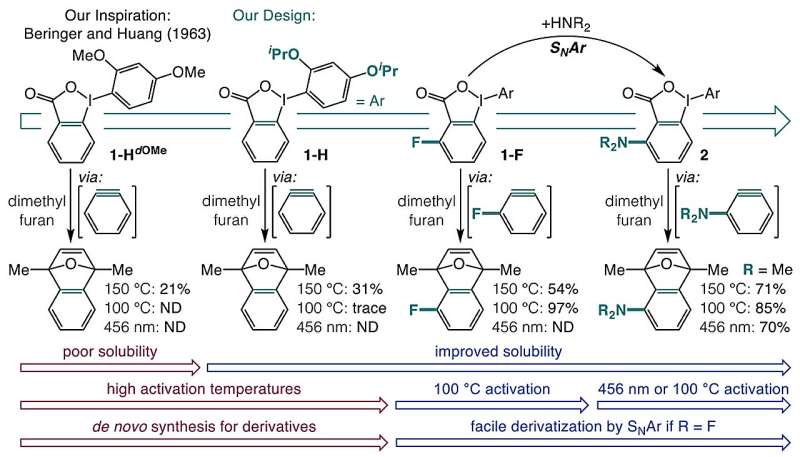
A team of researchers from the University of Minnesota has developed a groundbreaking one-pot method to synthesize blue light-responsive aryne precursors from commercially available carboxylic acids. This innovative approach, detailed in the journal Nature on November 11, 2025, enables the creation of aminated arynes and new aryne precursors in a single step, significantly enhancing the accessibility of these valuable organic intermediates.
Arynes, known for their highly reactive triple bonds within aromatic rings, play a crucial role in the synthesis of complex aromatic molecules, particularly in fields like drug discovery and agricultural chemistry. Despite their potential, the intricate process of designing arynes has traditionally deterred synthetic chemists from utilizing them. The new method simplifies this process, allowing for the easy derivation of arynes, which could transform synthetic chemistry practices.
Overcoming Synthetic Challenges
Building aromatic compounds with multiple substitutions is a fundamental yet challenging task in synthetic chemistry. Arynes, with their two reactive ends, facilitate various chemical transformations but have remained underutilized due to the limitations of existing synthesis methods. Traditional approaches often require harsh conditions, such as strong bases to strip protons from C–H bonds, followed by halide elimination, making them unsuitable for sensitive molecules.
Prior attempts to create arynes using thermally activated precursors have faced issues of instability, while UV-light methods frequently led to unwanted reactions. This landscape created a pressing need for a milder and simpler method for generating diverse aryne derivatives from readily available starting materials.
A New Approach to Aryne Synthesis
The research team, led by Chris M. Seong, identified that the solution to synthesizing arynes might lie in a simple reagent: o-iodoniobenzoates. They focused on converting these compounds into aryne precursors that could be activated using visible light or mild heat. Initially, the o-iodoniobenzoates posed challenges due to their poor solubility and propensity for side reactions.
Through extensive experimentation, the researchers discovered that introducing isopropoxy groups to these precursors not only improved solubility but also minimized unwanted reactions. This modification allowed the formation of aryne precursors that can be activated by blue light or heating to 100 °C. The study revealed that the heat activation process is largely driven by a field effect, where adjacent chemical groups enhance decarboxylation. In contrast, blue light activation at 398 nm excites the molecule to a triplet state, leading to the release of carbon dioxide and resulting in aryne formation.
The significance of this one-pot method lies in its compatibility with various functional groups, paving the way for simplified synthesis of complex aromatic compounds. This advancement not only facilitates advancements in pharmaceuticals and agrochemicals but also opens new avenues for exploration in previously uncharted chemical spaces.
The findings underscore the potential of this novel approach, which could revolutionize the way chemists synthesize aromatic compounds, ultimately contributing to innovations in multiple scientific fields. The research has been thoroughly reviewed and fact-checked, ensuring its credibility within the scientific community.
This article, originally authored by Sanjukta Mondal and edited by Lisa Lock, is part of a commitment to delivering accurate and independent science journalism. For more information on the study, refer to the original publication in Nature (DOI: 10.1038/s41586-025-09830-1).
-

 Top Stories1 month ago
Top Stories1 month agoUrgent Update: Tom Aspinall’s Vision Deteriorates After UFC 321
-

 Health1 month ago
Health1 month agoMIT Scientists Uncover Surprising Genomic Loops During Cell Division
-

 Science4 weeks ago
Science4 weeks agoUniversity of Hawaiʻi Joins $25.6M AI Project to Enhance Disaster Monitoring
-

 Top Stories1 month ago
Top Stories1 month agoAI Disruption: AWS Faces Threat as Startups Shift Cloud Focus
-

 Science2 months ago
Science2 months agoTime Crystals Revolutionize Quantum Computing Potential
-

 World2 months ago
World2 months agoHoneywell Forecasts Record Business Jet Deliveries Over Next Decade
-

 Entertainment1 month ago
Entertainment1 month agoDiscover the Full Map of Pokémon Legends: Z-A’s Lumiose City
-

 Top Stories2 months ago
Top Stories2 months agoGOP Faces Backlash as Protests Surge Against Trump Policies
-

 Entertainment2 months ago
Entertainment2 months agoParenthood Set to Depart Hulu: What Fans Need to Know
-

 Politics2 months ago
Politics2 months agoJudge Signals Dismissal of Chelsea Housing Case Citing AI Flaws
-

 Sports2 months ago
Sports2 months agoYoshinobu Yamamoto Shines in Game 2, Leading Dodgers to Victory
-

 Health2 months ago
Health2 months agoMaine Insurers Cut Medicare Advantage Plans Amid Cost Pressures








